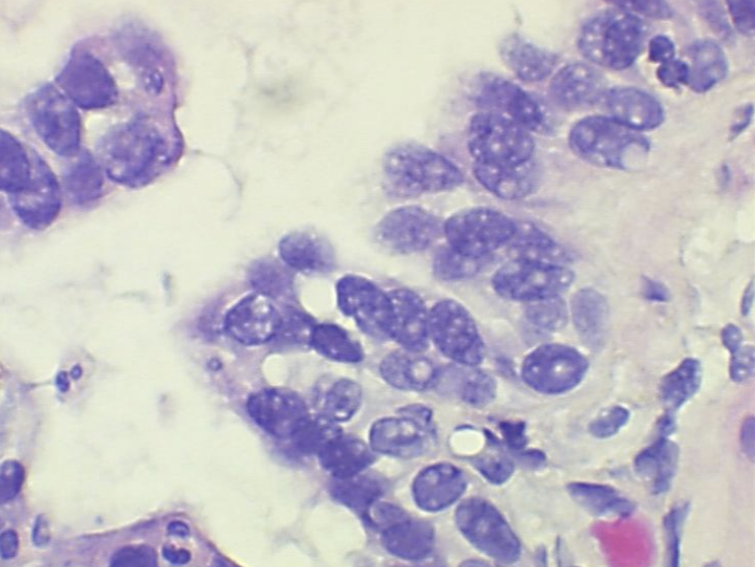
 Nuclear bubbling in histology refers to when nuclear detail is lost and nuclei appear “smudgy”, for lack of a better term. Neutral buffered formalin (NBF), though widely the most commonly used fixative in the modern histology lab, is recognized to have short comings when it comes to nuclei. Not leaving adequate time for fixation is one of the primary reasons for nuclear bubbling. NBF and other aldehyde fixatives work by creating chemical bonds between proteins in the tissue, what is known as cross-linking.
Nuclear bubbling in histology refers to when nuclear detail is lost and nuclei appear “smudgy”, for lack of a better term. Neutral buffered formalin (NBF), though widely the most commonly used fixative in the modern histology lab, is recognized to have short comings when it comes to nuclei. Not leaving adequate time for fixation is one of the primary reasons for nuclear bubbling. NBF and other aldehyde fixatives work by creating chemical bonds between proteins in the tissue, what is known as cross-linking.
This provides structural stability to the cell. If adequate time is not given for fixation when using NBF the nuclear proteins don’t have enough time to develop stable cross links. NBF generally requires at least 6-8 hours of fixation before processing, with ideal fixation time being 24-48 hours. In today’s lab with the emphasis on turnaround times, 24-48 hours of fixation may be unrealistic, but that 6-8 hours is very important. Anything below that will under-fix the tissue, and when the tissue is under fixed when processing starts, the alcohol in the processing steps will try to finish the job of stabilizing the proteins.
This is a problem because the proteins will start to essentially flip themselves inside out to accommodate the change in charge. Regular proteins in your body will organize with the charged amino acids towards the outside of the tangled ball of amino acids that make up protein, so that they can bond with the water in your body. During formalin fixation, this set up is preserved with uncharged amino acids on the inside and charged on the outside, which allows the positively and negatively charged dye to bind with the charged amino acids on the outside of the tissue when applied after fixation. In alcohol fixation, where there is no water, the charged amino acids try to “hide” on the inside.
Here the positive and negative amino acids bind with each other and hold the protein together in this inside out shape. Since the outside is uncharged, the dye is not as able to bind with the tissue, which is why alcohol fixation is not ideal for all stains.
In formalin fixation, proper fixation time is essential so the formaldehyde has enough time to come in and create all of the crosslinks it needs to make the structure stable and keep the charged amino acids on the outside. When your tissue is under-fixed in formalin, the alcohol will try to finish fixation during processing and end up flipping some of the proteins inside out.
Another major cause of nuclear bubbling is heat. You want to make sure that you drain the water from underneath the slides before putting them into an oven. Water underneath a section will overheat in high oven temperatures causing the nuclei to bubble. The same can happen if you put a freshly cut section directly on a heat source such as a hot plate. Try draining the water or air drying your slides in front of a fan before putting them in the oven to reduce water.
Resources:
Post on this topic in the NSH Block member community.
HQIP Nuclear Bubbling Fact Sheet
https://www.nsh.org/viewdocument/nuclear-bubbling-its-about-time
#2020#Blog#GeneralAnatomicPathology